Highlights
- Innovative REE Separation Techniques: Dr. Santa Jansone-Popova and colleagues at Oak Ridge National Laboratory highlight the critical need for environmentally sustainable rare earth element (REE) separation methods, focusing on neutral organic compounds to reduce ecological impacts.
- Advances in REE Separation: The Oak Ridge team explores how neutral organic compounds, like hydrocarbons and ethers, can improve selectivity across REE types, representing a promising pathway for eco-friendly REE separation technologies.
- Future Research Directions: The authors call for further investigation into areas like hydrochloric acid media, N-heterocycle ligands, and machine learning for ligand discovery to advance sustainable rare earth separation practices.
Santa Jansone-Popova, PhD (opens in a new tab), a chemist in the R&D staff and colleagues at Oak Ridge National Laboratory (opens in a new tab), come together in a paper to report that the mining, separation, and processing of rare earth elements (REE) pose significant environmental challenges. Serious ecological damage can ensue from what the authors employed by the U.S. federal lab refer to as traditional separation processes. Hence, what Dr. Jansone-Popova, originally from the Baltics, and colleagues’ position is “the critical need for innovative techniques that reduce environmental impacts.”
Santa Jansone-Popova, PhD (opens in a new tab), Corresponding Author

Most certainly this is a theme here at Rare Earth Exchanges. Environmental breakthroughs via technology and process disruption represent a key pathway for U.S. REE initiatives. In this journal entry in European Journal of Inorganic Chemistry (opens in a new tab) the authors discuss recent advancements in rare earth separation technologies. Of interest for this group out of Oakridge, Tennessee is a focus on the role of neutral organic compounds.
These compounds are molecules composed mainly of carbon and hydrogen atoms with no net electrical charge, meaning they are neither acidic nor basic in nature. They are generally stable in both water and organic solvents due to their lack of ionizable groups, and they do not readily donate or accept protons (H⁺ ions). Common examples of neutral organic compounds include alkanes, alkenes, alkynes, ethers, esters, and ketones.
Neutral organic compounds are commonly used as solvents, fuels, and intermediates in chemical synthesis. For instance, hydrocarbons serve as fuel sources (e.g., propane and butane), while ethers and esters are used as solvents in pharmaceuticals and paints due to their stability and relatively low toxicity. Understanding the behavior and properties of neutral organic compounds is essential in organic chemistry, as it allows chemists to predict reactions and design processes without the interference of acidic or basic groups.
And this brings us to the Oak Ridge National Laboratory review, exploring how these compounds change selectivity across the rare earth series, offering promising strategies for designing more effective rare earth element separation systems. This refinement and optimization likely represent the future of REE separation.
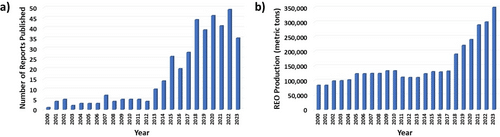
a) The annual count of reports published since 2000, determined by searching topics such as “extraction, separation, rare earths, rare earth elements” on the Web of Science database. b) The global annual production of rare earth oxides since 2000.
The authors also emphasize other areas requiring additional investigation to improve the sustainability of these critical processes.
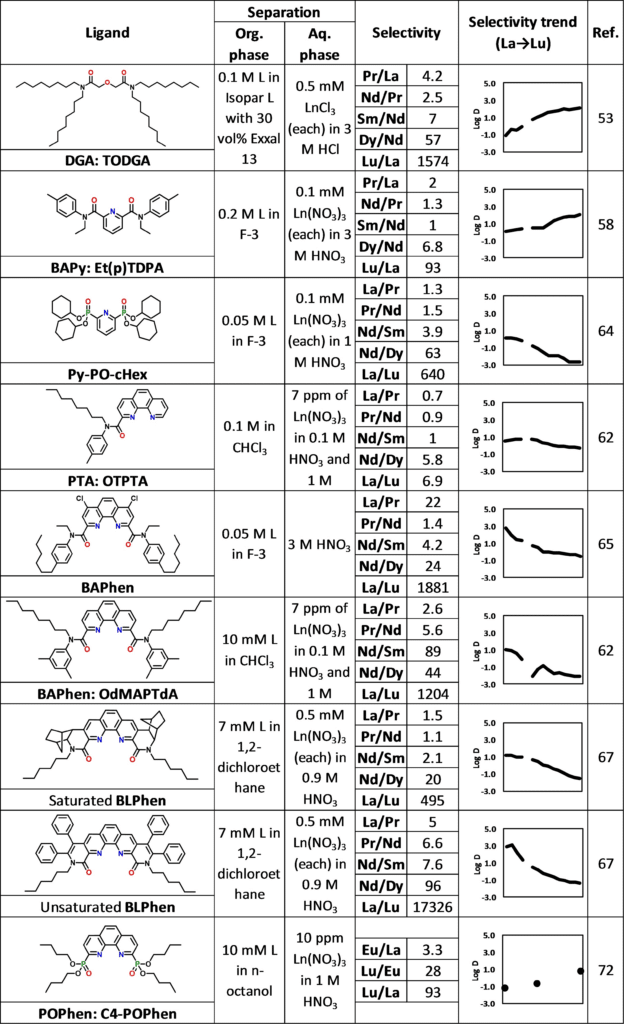
Examples of neutral ligands used as extractants to separate rare earth elements.
Has the field of REE separation witnessed significant advancements in the past three years?
Yes. This encompasses selective crystallization, selective dissolution, the application of efficient N-heterocycle-based extractants in solvent extraction processes, and the introduction of dual-ligand separation systems
What are key areas that warrant further attention and would contribute to the broader research community?
| Research Areas | Summary |
|---|---|
| Reporting Distribution Values | Authors in this field are encouraged to provide distribution values, either in the main text or as part of the Supporting Information file. Access to these data is important and would eliminate the need for software use to extract numerical values from figures, reducing the risk of data misrepresentation. |
| Exploration of Hydrochloric Acid/Chloride Media | While nitric acid media are commonly used in REE studies, particularly influenced by nuclear research, there is a need to explore separation processes using hydrochloric acid/chloride media. This would be of great interest to the industry, considering high price and the strong oxidizing nature of both nitric and perchloric acids. |
| Stability and Production of N-Heterocycle-Based Ligands | The development of new N-heterocycle-based ligands for REE separation is flourishing. The stability of phenanthroline-based complexants in aqueous acidic environments needs thorough investigation. Phenanthroline-based complexants involve a multi-step synthesis starting from relatively expensive commercially available chemicals. Better synthetic methods/process chemistry is needed to overcome cost challenges to compete with state-of-the-art phosphorous-based acids. |
| Water-Soluble RE3+ Complexants in Dual-Ligand Systems | The use of water-soluble RE3+ complexants in two-ligand separation systems requires extensive work for maturing this technology in REE separation. The primary focus should be on identifying means to recover and recycle the ligand continuously in the RE separation process. |
| Bridging Fundamental Research and Applied Efforts | While new ligands and separation methods are driving innovation in RE separations field, there is a need to bridge the gap between fundamental and applied research. Efforts in conducting multistage RE separations, or larger scale demonstrations, would be of great interest to the community. |
| Leveraging Machine Learning (ML) for Ligand Discovery | The use of ML methods and predictive models to discover new ligands for improved REE separation has gained momentum and should be further leveraged to advance the field. |

0 Comments