Highlights
- Professor Koen Binnemans' SOLVOMET group published a thermodynamic model for acid extraction using TEHA-octanol.
- The model achieves 3.74% RMSE accuracy in predicting recovery of H₂SO₄, HCl, and methanesulfonic acid from industrial leach solutions.
- The predictive framework captures synergistic solvent behavior that has challenged process engineers.
- This technology enables digital twin simulations to optimize critical mineral recovery circuits before expensive pilot-scale construction.
- Acid recycling through this technology reduces reagent costs and neutralization waste.
- The approach strengthens the economic viability of circular hydrometallurgy for nickel, cobalt, and critical metals as ore grades decline.
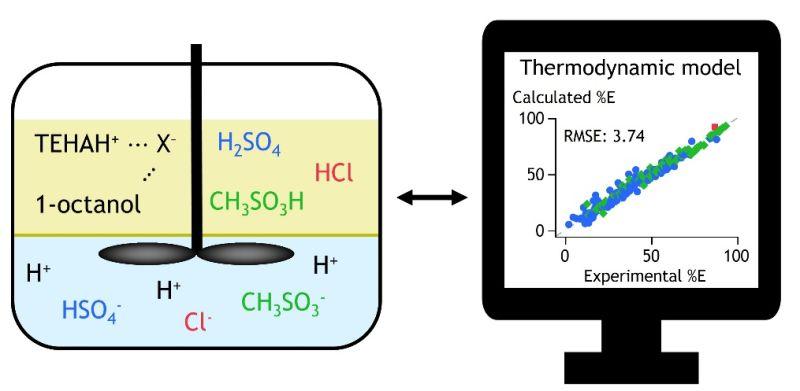
When Professor Koen Binnemans (opens in a new tab) of KU Leuven posts research, industry pays attention. His SOLVOMET group’s (opens in a new tab) latest paper—co-authored with Rayco Lommelen (opens in a new tab)—dives deep into a niche that underpins the economics of rare earth and critical mineral recovery: the thermodynamics of acid extraction. Their model, published in Reaction Chemistry & Engineering (2025), tackles one of hydrometallurgy’s least glamorous but most consequential problems—how to recover and recycle acids efficiently instead of neutralizing them into waste.
Table of Contents
A Quiet Revolution in the Solvent Phase
At the heart of the study lies tris(2-ethylhexyl)amine (TEHA) paired with 1-octanol, a duo long known to extract acids like H₂SO₄, HCl, and CH₃SO₃H (methanesulfonic acid) with impressive efficiency. What’s new here isn’t chemistry—it’s predictive power. Using OLI Systems’ Mixed-Solvent Electrolyte (MSE) model, the SOLVOMET team developed a thermodynamic framework that finally captures the messy, synergistic behavior of TEHA–octanol extraction systems.
That synergy—where the solvent pair acts as more than the sum of its parts—has long stumped process engineers. Binnemans’ model bridges that gap, linking molecular interactions with bulk extraction performance. Validation against NiSO₄-containing leach solutions showed tight correlation between prediction and experiment (RMSE 3.74%), suggesting real-world applicability.
From Lab Insight to Supply Chain Impact
Why should investors or policymakers care about acid balance sheets? Because acid recycling is the hidden lever of circular hydrometallurgy. Every liter of sulfuric acid recovered instead of discarded means less reagent cost, lower neutralization waste, and a tighter loop between leaching and refining—key to making rare earth, nickel, cobalt, and other critical-metal recovery both cleaner and cheaper.
In industrial ecosystems where feedstock grades are falling and environmental standards are rising, this work matters. The model could eventually underpin digital twin simulations for solvent extraction circuits, reducing pilot costs and optimizing plant design before steel ever meets concrete.
Measured, Rigorous, and Refreshingly Unsensational
There’s no hype here—just sound chemistry and meticulous modeling. Funded by the European Union’s ERC CIRMET project (No. 101093943), (opens in a new tab) this work strengthens Europe’s intellectual leadership in critical-material process science. It’s a reminder that progress in the rare earth supply chain doesn’t always come from new mines or flashy magnets—sometimes, it’s born in a beaker and perfected in an equation.
Source: Rayco Lommelen & Koen Binnemans, Reaction Chemistry & Engineering, 2025
©!-- /wp:paragraph -->

0 Comments